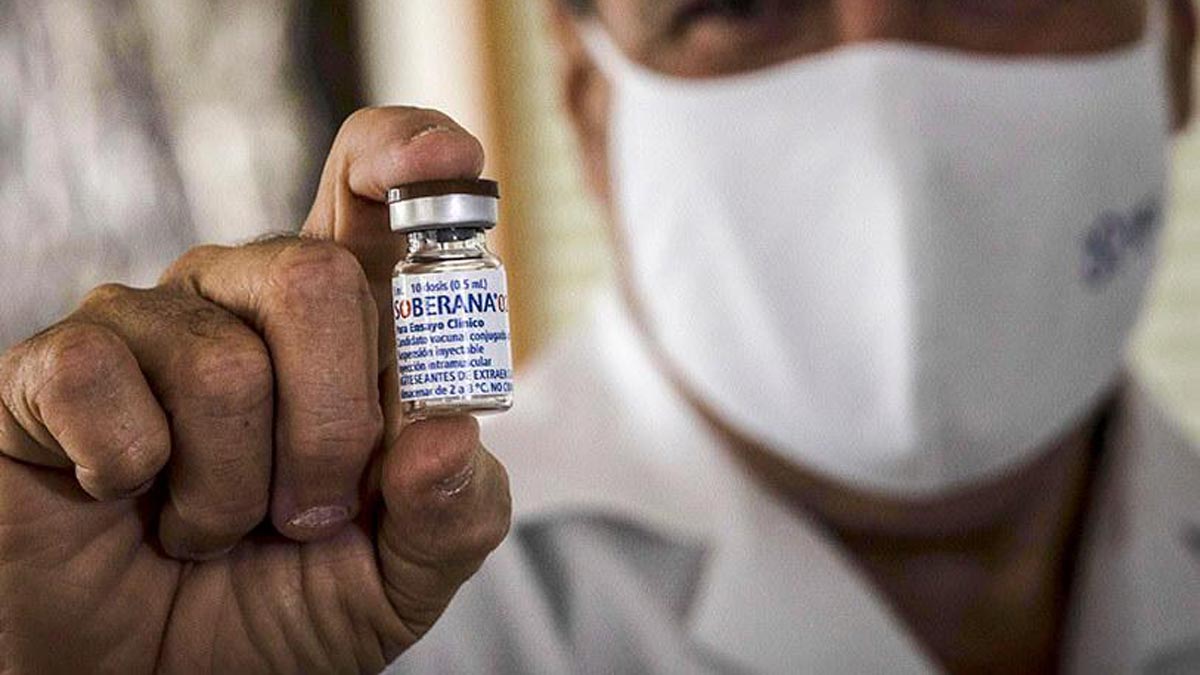
The Center for State Control of Medicines, Equipment and Medical Devices (CECMED) approved the emergency use of the SOBERANA 02 in the pediatric population aged 2 to 18 years of age. The manufacturer of the vaccine, the Finlay Vaccine Institute (IFV), had applied for approval.
CECMED approved the Soberana 02 vaccine based on the requirements of quality, safety, and immunogenicity for this group.
They based the approval on the trial results of an application of a two-dose schedule in children aged 3 to 18 years old and another in persons aged 19 to 80 years.
The results obtained in the clinical trials were superior in all variables in the group aged 19 to 80 years. It showed similar results in young adults aged 19 to 29 years.
The safety profile showed similar results between the groups compared. They include children from age 2 in the approval, based on information provided by the IFV.
CECMED carried out inspections of the clinical testing sites where the trials took place verifying compliance with Good Clinical Practices.

From our staff writers and editors.